Protoplast culture
- 2. Protoplast Culture: definition Isolated protoplasts have been described as "naked" cells because the cell wall has been removed by either a mechanical or an enzymatic process. In the isolated protoplast the outer plasma membrane is fully exposed
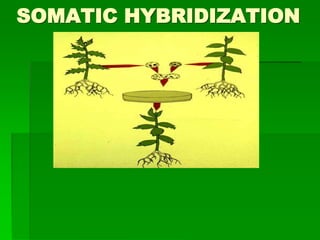
- 3. Development of hybrid plants through the fusion of somatic protoplasts of two different plant species/varieties is called somatic hybridization
- 4. Somatic hybridization technique 1. isolation of protoplast 2. Fusion of the protoplasts of desired species/varieties 3. Identification and Selection of somatic hybrid cells 4. Culture of the hybrid cells 5. Regeneration of hybrid plants
- 5. Isolation of protoplast from leaves Leaves are most commonly used Step are 1) Sterilization of leaves 2) Removal of epidermal cell layer 3) Treatment with enzymes 4) Isolation of protoplast Purification of protoplast Viability of protoplast
- 6. Isolation of Protoplast (Separartion of protoplasts from plant tissue) 1. Mechanical Method 2. Enzymatic Method
- 7. 1. Mechanical Method Plant Tissue Collection of protoplasm Cells Plasmolysis Microscope Observation of cells Cutting cell wall with knife Release of protoplasm Klercker 1892 (cited in Cocking 1972)
- 8. Suppose an animal or a plant cell is placed in a solution of sugar or salt in water. If the medium is hypotonic — a dilute solution, with a higher water concentration than the cell — the cell will gain water through osmosis. If the medium is isotonic — a solution with exactly the same water concentration as the cell — there will be no net movement of water across the cell membrane. If the medium is hypertonic — a concentrated solution, with a lower water concentration than the cell — the cell will lose water by osmosis. Plasmolysis is the process in plant cells where the cytoplasm pulls away from the cell wall due to the loss of water through osmosis. This occurs in ahypertonic solution
- 9. 1. Mechanical Method Used for vacuolated cells like onion bulb scale, radish and beet root tissues Low yield of protoplast Laborious and tedious process Low protoplast viability Restricted to tissues having large vacuolated cells.
- 10. 2. Enzymatic Method Leaf sterlization, removal of epidermis Plasmolysed cells Plasmolysed cells Pectinase +cellulase Pectinase Protoplasm released Release of isolated cells cellulase Protoplasm released Isolated Protoplasm (Cocking 1960) Isolation of protoplast from root tips of tomato (Lycopersicon esculentum)
- 11. Enzymes for protoplast isolation The enzymes that can digest the cell walls are required for protoplast isolation. Chemically plant cell – composed- cellulose, hemicellulose,, pectin So req- enzymes- cellulase, hemicellulose, pectinase In fact, the various enzymes for protoplast isolation are commercially available. The enzymes are usually used at pH 4.5 to 6.0, temperature 25-300C with a wide variation in incubation temp and that may range from half an hour to 20 hrs The enzymatic approach by two ways: a)Two step or sequential method- pectinase (to separate cells by degrading middle lamella and then cellulase removes the cell wall proper) b) One step or simultaneous method- Simultaneously both macroenzyme and cellulase
- 12. •Obtain sterile plant material •Rinsing in a suitable osmoticum •Facilitating enzyme penetration •Sequential or spontaneous •Purification of the isolated protoplasts (removal of enzymes and cellular debris) •Transfer to a suitable medium Use of enzymes results in a high yield of uniform protoplasts after removal of cellular debris Protoplasts can originate from different sources: greenhouse or field material, micropropagated plants, calli,
- 13. •Healthy leaves, removed from the plant and washed, sterilized and rewashed in sterile distilled water (subsequent procedures are conducted under aseptic conditions). When leaves are in the final rinse, lower epidermis is peeled from the leaves or the lower epidermis is scored several times. Cut the leaves into small sections, and transfer to filter sterilized enzyme solution. Seal the dishes wrap them with aluminum foil (leave overnight). Teased gently with forceps to release the protoplasts. Purify protoplasts (filtration, centrifugation, and washing)
- 14. Enzymatic Method Used for variety of tissues and organs including leaves, petioles, fruits, roots, coleoptiles, hypocotyls, stem, shoot apices, embryo microspores Mesophyll tissue - most suitable source High yield of protoplast Easy to perform More protoplast viability
- 15. •Protoplasts are filtered through a nylon mesh (64micrometer) to remove undigested tissue, cell clumps, and cell wall debris. •Transfer filtrate to centrifuge tube and spin at + 75 x g (5 min). •Debris (in supernatant) is carefully removed (protoplasts have formed a pellet at the base of the tube). •Protoplasts are carefully resuspended in culture medium (plus 13% mannitol), and the process is repeated three times. •Protoplasts are examined for density and viability. Protoplast purification
- 16. •Fluorescein diacetate: accumulates only inside the plasmalemma of viable protoplasts, can be detected with fluorescence/UV microscopy •Phenosafranine stainig: used at 0.01% conc and specific for dead protoplast that turn red. Viable protoplast remain unstained •Evans blue: Intact viable protoplasts, exclude the Evans blue stain. Impermeability of the cell to Evans blue indicates a living cell. Checking of viability of protoplast
- 17. Protoplast Culture The very first step in protoplast culture is development of a cell wall around the membrane of the protoplast. Protoplasts can been cultured in several ways: •Hanging-drop cultures •Microculture chambers •Soft agar (0.75 % w/v) matrix. This is one of the better methods as it ensures support for the protoplast.
- 18. Culture media A) Nutritional components : medium should be devoid of ammonium and quantities of iron and zinc should be less, The concentration of calcium should be 2-4 times higher than used for cell culture. This is needed for membrane stability. High auxin/kinetin ratio is suitable to induce cell division while high kinetin/auxin ratio is required for regeneration. Carbon source is normal as used and vitamins used for protoplast cultures are the same used in standard tissue culture media.
- 19. B) Osmoticum: Protoplast released directly into standard cell culture medium will burst Broadly refers to the reagents/chemicals that are added to increase the osmotic pressure of a liquid. Isolation and culture of protoplast require osmotic protection until they develop a strong cell wall. If freshly isolated protoplasts are directly added to culture medium they will burst. Osmoticum: 0.3 to 0.7 M of sugar solution., mannitol or sorbitol.
- 20. Culture methods Liquid culture Liquid Droplet method Hanging Droplet method Feeder layer Co-culturing
- 22. SWEET ORANGE SUSPENSION CULTURE PROTOPLASTS
- 23. RMAN
- 25. From an idea to generation
- 26. Protoplast Fusion (Fusion of protoplasts of two different genomes) 1. Spontaneous Fusion 2. Induced Fusion Intraspecific (Callus) Intergeneric Electrofusion Mechanical Fusion Chemofusion
- 27. 1.Spontaneous Fusion Protoplast fuse spontaneously during isolation process mainly due to physical contact • Intraspecific produce homokaryones • Intergeneric have no importance
- 28. 2. Induced Fusion A) Chemofusion - fusion induced by chemicals • Types of fusogens • PEG • NaNo3 • Ca 2+ ions • Polyvinyl alcohol • Lysozyme • Dextran • Fatty acids and esters.
- 29. Of these , the following treatments have yielded success in producing somatic hybrid plants. 1 NaNOӡ treatment : first demonstrated by power et al.(1970) Isolated protoplasts cleaned by floating in 10% sucrose solution . Transfer of protoplast in 5.5% NaNOӡ solution incubation then centrifugation @2000rpm protoplast kept in water bath for 300C for 30 min during which fusion occurs This treatment results in low frequency of heterokaryon formation , particularly when mesophyll protoplast are used.
- 30. 2. High pH and high Ca++ treatment : Method was developed by Kelier and Melchers(1973) for fusing two different lines of tobacco protoplast and now commonly used. Isolated protoplast incubated in solution of 0.5M mannitol containing 0.05M CaCl2, pH 10.5 and temp. 37ºC (30-40 min.) aggregation of protoplast and fusion usually occurs with in 10 min. By this methods , 20-50% of the protoplasts involved in fusion.
- 31. 3. PEG treatment : This has the become the method of choice, due to its high success rate. Kao and Michayluk (1974). Isolated protoplasts in culture medium (1ml) are mixed with equal volume (1ml) of 28-56% PEG in a tube. PEG enhances fusion of protoplasts in several species. tube is shaken(5 min) and allowed to settle(10 min). settled protoplasts washed several times with culture medium. This method is widely used for protoplast fusion. Advantage : a) high frequency of heterokaryon formation b) low toxicity to cells. c) reduced formation of binucleate heterokaryon
- 32. Mechanism of fusion Protoplast fusion: PEG a) Agglutination (Adhesion)
- 33. Schematic representation of 3 most successful protoplast fusion strategies. Protoplast of species(A) + Protoplast of species(B) ( suspended in enzyme mixture) PEG induced fusion Electrofusipn High Ca 2+ High pH treatment PEG(28-58%) (high MW)(inc. agg.) Low voltage 15-30 min. Protoplast aggregation Protoplast Ca 2+ 50mM/1 chain formed , centrifuge- 3min, 500 rpm pH 10.5, temp.37˚C. Washing medium High Voltage i 40-50 min incubation pH 9-10 ( few micro second) Ca 2+ 50mM/1 water bath Protoplast fusion
- 35. induced fusion contd…. B) Mechanical Fusion- Physical fusion of protoplasts under microscope by using micromanipulator and perfusion micropipette. C) Electrofusion- Fusion induced by electrical stimulation. • Pearl chain of protoplasts is formed by low strength electric field (10kv m-1) • Fusion of protoplasts of pearl chain is induced by the application of high strength electric field (100kv m-1) for few microsecond. • So the plasma lemma distribution and organization disturbances leading to the fusion of two protoplasts and the fusion of these protoplast is done in device electroporator.
- 36. Protoplast fusion: Electrofusion Low intensity AC current to protoplast suspension 750-1000 V/cm for short duration 20-50 µsec
- 37. 2.2 Protoplast fusion: Electroporation
- 38. Selection of hybrid cells Protoplast suspension recovered after a treatment with a fusion inducing agent (fusogen) consists of following cell types : - 1) Unfused protoplast of two species / strain. 2) Products of fusion between two or more protoplasts of the same species(homokaryon). 3) Hybrid protoplasts produced by fusion between one (or more) protoplast (s) of each of the two species.(heterokaryon). Therefore , a specific strategies has to be employed for their identificaton and isolation. This step is called Selection of hybrid cells.
- 39. Identification and Selection of somatic hybrid cells Hybrid identification- Based on difference between the parental cells and hybrid cell with respect to • Pigmentation • Cytoplasmic markers • Fluorochromes like FITC (fluoroscein isothiocyanate) and RITC (Rhodamine isothiocyanate) are used for labelling of hybrid cells(0.5 mg/l prior incubation time) • Presence of chloroplast • Nuclear staining • Heterokaryon is stained by carbol-fuschin, aceto- carmine or aceto-orcein stain
- 40. Hybrid Selection (Several markers are used ) • Genetic complementation • Phytotoxins • Specific amino acid • Auxin autotrophy • Antibiotics • Auxotrophic and metabolic mutants • Chromosomal analysis • Herbicides
- 41. Culture of the hybrid cells Hybrid cells are cultured on suitable medium provided with the appropriate culture conditions.
- 42. Regeneration of hybrid plants Plants are induced to regenerate from hybrid calli . These hybrid plants must be at least partially fertile, in addition to having some useful property, to be of any use in breeding schemes.
- 43. Somatic hybrid- two types : 1) Symmetric hybrids : Some somatic hybrid plants retain the full somatic complements of the two parental species; these are called Symmetric hybrids . 2) Asymmetric hybrids: Many somatic hybrids exhibit the full somatic complement of one parental species while all or nearly all chromosomes of the other parental species are lost during the preceding mitotic divisions, such hybrid are referred as Asymmetric hybrids.
- 44. Advantages of somatic hybridization Production of novel interspecific and intergenic hybrid Pomato (Hybrid of potato and tomato) Production of fertile diploids and polypoids from sexually sterile haploids, triploids and aneuploids Transfer gene for disease resistance, abiotic stress resistance, herbicide resistance and many other quality characters
- 45. Advantages of somatic hybridization Production of heterozygous lines in the single species which cannot be propagated by vegetative means Studies on the fate of plasma genes Production of unique hybrids of nucleus and cytoplasm
- 46. Limitations of Somatic hybridization Poor regeneration of hybrid plants Non-viability of fused products Not successful in all plants. Production of unfavorable hybrids Lack of an efficient method for selection of hybrids No confirmation of expression of particular trait in somatic hybrids
- 47. NEW SOMATIC HYBRID PLANT
- 48. Cybrids Cybrids are cells or plants containing nucleus of one species but cytoplasm from both the parental species .
- 50. Cybrid of “Murcott” (the Honey tangerine) containing the mtDNA CMS of G1 Satsuma mandarin
- 51. The End Thank you for your Attention 51
- 52. Easy to start Challenging to grow Start HereKeep in mind the “Indian context” Understanding regulation is critical Building the right team is critical
- 53. Power of idea is great but it’s all about execution Most obvious is the most hidden Don’t get married to your ideas Find what sells over and over again Don’t presume Get feedback early on
Hinweis der Redaktion
- Fda accumulates within 5 min . Fda is dissolved in acetone and used at a conc of 0.01%. Protoplast treated with Fda must be examined within 5-15 min after staining as FDA dissociates from the membrane after 15 min
