Formulation & Evaluation of Ocular Controlled Drug Delivery Systems
- 1. FORMULATION & EVALUATION OF OCULAR CONTROLLED DRUG DELIVERY SYSTEM Presented by Saiesh phaldesai
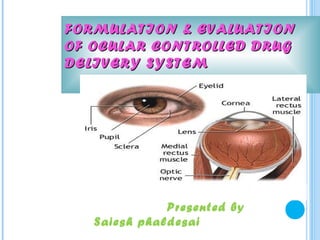
- 2. Human eye has a spherical shape with a diameter of 23mm cornea , lens , viterous body not have blood suply Oxygen and nutrients are supplied by aqueous humour Cornea is formed by criss crosing layer of collagen and bounded by elastic laminae
- 3. EYE BALL The wall of the human eye (globe) is composed of concentric layer; The outer fibrous layer. A middle vascular layer : Uvea tract - choroid, cilliary body, and iris. A nervous layer-the retina.
- 4. The sclera consist of tough fibrous layer Both cornea and sclera withstand the intraocular tension maintained in the eye The eye is constantly cleansed and lubricated by the lacrimal appartus 1. lacrimal gland 2. lacrimal canal 3. lacrimal sac 4. nasolacrimal duct
- 5. COMPOSITION OF TEAR The secretion is a clear, watery fluid containing numerous salts, glucose, other organic compounds, approximately 0.7% protein and the enzyme, lysosome. Water-98.2% Organic Protein0.67% Solids-1.8% element Sugar-0.65% Nacl-0.66% Urea-0.03%
- 6. FORMULATION OF OCULAR DELIVERY SYSTEM The conventional ocular dosage form for delivery of drugs are : I. Eye drops II. Eye ointments III. Eye suspension
- 7. Eye drops : - Advantages : most prescribed dosage form easy to instill Disadvantage : rapidly drained away 1.2% bioavailabilty large fluctuation in intraocular drug level get diluted in the tear freq . adm’n needed to maintain sustained level limited corneal permeability very dose is reqd 7
- 8. Eye ointments : - Advantages : larger contact time and greater bioavailability although slower onset and time of peak absorption .
- 9. Eye suspension : - Advantages : longer duration of action Disadvantages : impredictable release change in drug level due to const inflow and outflow of lacrimal fluid this effects drug dissolution from insoluble drug particles
- 10. Approaches made towards optimization of ocular delivery systems: 1) Improving ocular contact time 2) Enhancing corneal permeability 3) Enhancing site specificity
- 11. REQ UISITE OF CONTROLLE D DR UG DELIVERY SYSTEM 1) To overcome the side effects of pulsed dosing (frequent dosing and high concentration) produced by conventional systems. 2) To provide sustained and controlled drug delivery. 3) To increase the ocular bioavailability of drug by increasing corneal contact time. This can be achieved by effective coating or adherence to corneal surface. 4) To provide targeting within the ocular globe so as to prevent the loss to other ocular diseases.
- 12. 5) To provide comfort and compliance. 6) To provide the protective barriers like drainage, lacrimation and diversion of exogenous chemicals into systemic circulation by conjuctiva. 7) To provide the better housing of delivery system in eye. 8) To provide prolonged drug release.
- 13. FACTORS INFLUENCING OCULAR BIOAVAILABILTY 1. Dilution of drug soln 2. Nasolacrimal drainage 3. continuous inflow & outflow of lacrimal fluid also cause loss of drug 4. The lacrimal fluid constituent like proteins degrade the drug 5. Productive and non productive absorption of topically applied drug
- 14. THE FOLLOWING RECENT TRENDS OF DOSAGE FORMS ARE IN VOGUE: 1) Controlled ocular delivery systems: 1. Polymeric solutions 2. Phase transition systems 3. Mucoadvesive/ bioadhesive dosage forms 4. Collagen shields 5. Pseudolatices 6. Ocular penetration enhancers 7. Ocular iontophoresis.
- 15. 2) Ocular drug delivery devices: 1) Matrix-type drug delivery systems. i) Hydrophilic soft contact lenses ii) Soluble ocular inserts iii) Scleral buckling materials 2) Capsular-type drug delivery systems. i) Ocuserts and related devices ii) Implantable silicone rubber device 3) Implantable drug delivery pumps. i) Osmotic minipump and implantable infusion system 4) Others. i) Ocufit & lacrisert ii) Minidisk ocular therapeutic system iii) New ophthalmic delivery system.
- 16. CONTROLED OCULAR DELIVERY SYSTEM A) Polymeric solution: the addition of polymers like methyl cellulose , poly vinyl alcohol, hydroxy propyl cellulose and poly vinyl pyrolidine to the eye drop solutions increases the corneal penetrations of drugs. This is presumably due to an increase viscosity, which decrease the rapid initial drainage rate, increase corneal contact time and thus sustains to some extent the initial tear concentration of drug
- 17. B) Phase transition systems: these are the liquid dosage forms which shift to the gel or solid phase when instilled into the cul-de-sac. There are three types of phase transition systems: i) Temperature dependent phase transition systems: polymers that are normally used are lutrol FC-127 and poloxamer 407, ii) pH triggered phase transition systems: cellulose acetate phthalate and carbopol iii) Ion-activated systems: gelrite and gellan is a new phase transition system
- 18. C) Mucoadhesive/ bioadhesive dosage forms 1) A good bioadhesive should exhibit a near zero contact angle to allow maximal contact with the mucin coat. 2) To diffuse and penetrate into mucin layer, flexibility in chain of polymer is required. 3) An increase in molecular weight to a critical value, increases the bioadhesion. 4) pH and ionic strength of dosage from also affects the bioadhesion performance
- 19. Increases the corneal contact time water soluble polymers face the disadvantage of having a short half life. The adhesion often detaches itself from the rate controlling drug delivery device and caused a premature release of drug. Ex- . polycarbophil exhibits strongest bioadhesion at an acidic pH, Hydroxy propyl cellulose(HPC), polyacrilic acid, PEG, dextrans, hyaluronic acid, polygalactouronic acid, xyloglucon etc
- 20. D)Collagen Shields: For the drug delivery, the shields are rehydrated in water solution of drug, where the drug is absorbed by the protein matrix and is released once the shield dissolves in eye. As the dissolution time for the cross linked collagen shields are longer than those of the non cross linked type they might be useful ocular delivery devices because they can allow to achieve higher drug concentration in cornea and aqueous humor. Drawbacks: Application of shield requires anaesthetize the cornea. Often produce some discomfort.
- 21. E) Pseudolattices: are a new class of polymeric colloidal dispersions and film forming agents . Before instillation of organic solution of polymer, by applying vacuum or by using controlled temperature, to remove water partially and to an extent that residual water is sufficient to disperse in an aqueous phase to form a o/w type emulsion. Such dispersions are referred as a pseudolattices. The drug from such systems is released slowly over a prolonged period of time ensuring better ocular availability and patient compliance by avoiding frequent instillation of preparation.
- 22. F) Ocular Penetration Enhancers: like actin filament inhibitors, surfactants, bile salts, chelators and organic compounds have been used to increase the bioavailability of topically applied drugs Drawback: Tissue irritation and damage. G) Ocular Iontophoresis: is a process in which the direct current drives ions into the cell or tissues
- 23. MATRIX – TYPE DRUG DELIVERY SYSTEM 1.Hydrophilic soft lenses: These are easy to fit, well tolerated, & hydrophilic soft contact are more popular for correction of hydro gel like PHP (helifcon-A) co-polymer(80% of 2-hydroxy- ethylmethacrylate and 20% of N-vinyl-2-pyrolidone). 16mm - diameter, 0.3mm - thickness, 40% to 45% - hydration.
- 24. These lenses are presoaked in prednisolone sodium phosphate -20min they were able to maintain aqueous and corneal level 2-3 times higher at 4hr, than the level obtained after topical administration of plain predinsalone soln . Idoxuridine , polymixin B, pilocarpine 24
- 25. 2.Soluble ocular insert : These ate thin, elastic, oval plates and made from polymer (PVA) and co-polymer of polyamide, ethylacrylate and vinyl pyrrolidine. when SODIs inserted into a conjunctival sac. It absorbs tears rapidly, swells and dissolves-30-90min and release the drug in a controlled manner. Eg : hydrocortisone 10 mg SODI
- 26. 3. Scleral buckling materials : These are used in retinal detachment surgery as they causes post operational infection . So to prevent this complication, scleral buckling material can be made to absorb an antibiotic. ₪ Gelatin film ₪ Solid silicon rubber
- 27. CAPSULAR - TYPE DRUG DELIVERY SYSTEM 1.Ocusert and related device : The system consists of a pilocarpine and alginic acid are sandwiched between 2 thin transparent-rate controlling ethylene-vinyl acetate co-polymer membrane. A retaining ring of same material impregnated with titanium dioxide encloses the drug resorvoir. Eg : pilo -20 , pilo -40
- 28. 1.Ocusert and related device : Drug release controlling polymer Titanium dioxide ring Drug reservoir Polymer membrane
- 29. 2.Implantable silicone rubber device : By using this device hydrophobic drug can deliver at a constant release rate. BCNU (1,3bis{-2-chloro ethyl}-1-nitrosurea) is an intraocular malignancy agent. It consists of 2 sheet of silicone rubber glued together only at the edges with silicone adhesive, which release the drug at constant rate about (200- 400µg/h).
- 30. IMPLANTABLE DRUG DELIVERY SYSTEM Osmotic mini pump & Implantable infusion system : The osmotic mini pump is a useful implantable drug delivery system with a constant drug delivery rate with a pumping duration of up to 2 weeks. The implantable infusion system, the pumping force is generated by an expanding fluid (a fluorocarbon in liquid gas equilibrium) at body temperature.
- 31. OTHER DELIVERY DEVICES OCUFIT: Sustained release, rod shaped device made up of silicone elastomer and these are designed to fit the shape and size of the human conjunctional. LACRISERT: Cylindrical device, which is made of cellulose and used to treat dry eye patients. These devices have a long retention (2-weeks or more) and sustained release.
- 32. MINIDISK OCULAR THERAPEUTIC SYSTEM It is a monolithic polymer device, shaped like miniature contact lens, with a convex and concave faces. The device can easily be oxygen permeabile because of its particular size and shape. Advantage: Requires less time and less manual skill for insertion, when compared with lacrisert.
- 33. New ophthalmic delivery system (NODS): is a method of presenting drugs to the eye with a water soluble drug loaded film. It provides for accurate, reproducible dosing in an easily administered preservative form Microsphere / nanosphere : Poly alkyl cyano acrylate (PACA) Poly E caprolactone (PECA) eg or drugs – metipranolol , betaxalol decrease particle size increase bioavailability
- 34. Liposomes : Advantages – controlled release more favorale for hydrophobic drug protection from ocular enzymes non toxic non irritant intimate contact with the cornea increased permiabilty eg – idoxuridine , pen-G , ephenephrine , cyclosporin
- 35. Niosomes : dialkyl polyoxyetylene ether , cholestrol vesicles Advantages : stable for both hydrophillic and hydrophobic non toxic bio–degradable biocompatable non immunogenic improved bioavailabilty CR and targetting
- 36. SOFT DRUG SYSTEMS (SDS) Active biological substance which are deactivated in a predictable and controlled way after they achieve there therapeutic role 1) Soft analogues – closely structural similarality with metabolite 2) Active soft compounds – inactive compd + pharmacophore eg – N – choloramine antimicrobials 3) Inactive metabolite – inactive metabolite converts to parent drug analogue which after action again converted into inactive eg – sds of atropine
- 37. 4) Controlled release of endogeneous soft compds – harmones and neurotransmitter are the natural SDS Eg for SDS – soft steroids ,beta adrenargic blocker , prostaglandin
- 38. EVALUATION OF OPHTHLMIC INSERT Physico-chemical properties: Moisture Absorption (%) Moisture loss (%) Thickness (mm) Weight Variation (mg) Folding Endurance Drug Content- In-vitro kinetics In-vivo release studied
- 39. INVIVO STUDIES For the study purpose : Rabbits weighing 2-2.5 kg, they fed—standard diet. One drug-ophthalmic insert-right eye-test. Blank-left eye-control (cul-de-sac). At regular time interval remaining ocular insert were removed and analyze for the drug content by using uv-visible spectrophotometer. Drug released Initial drug Drug content content at any content after removal time before placing of occusert the ocusert from eye
- 40. PILOCARPINE DRUG DELIVERY SYSTEM It is the chief alkaloid extracted from the leaf lets of pilocarpus jaborandi and pilocarpus microphyllus. Highly preferred---ocular hypotensive agent --- decrease intra ocular pressure. Effect--cholinomimetic,
- 41. BIOPHARMACEUTICS OF OCULAR PILOCARPINE ADMINISTRATION 1. Dilution of drug soln 2. Nasolacrimal drainage 3. continuous inflow & outflow of lacrimal fluid also cause loss of drug 4. The lacrimal fluid constituent like proteins degrade the drug 5. Productive and non productive absorption of topically applied drug
- 42. PRECORNEAL DISPOSITION OF DRUGS lacrimal fluid Lacrimal Occular gland delivery Precorneal Corneal cavity epithelium Lacrimal canal stroma Conjuctival Lacrimal uptake Aq.humor sac Nasolacrimal duct Elimination Systemic Circulation
- 43. Disposition of drug take place by 1. Nasolacrimal drainage (80 %) 2. Tear turn over 3. Productive corneal absorption 4. Non productive conjunctival uptake nasolacrimal drainage removes the drug untill the volume reaches to normal residual tear volume (7.5 micro lt) Smaller the instilation volume better is the bioavailabilty
- 44. TRANSCORNEAL PERMEATION OF PILOCARPINE 1. The existence of a permeation barrier in the lipophilic corneal epithelium 2. Uptake and permeation of drug through cornea are rapid process 3. Transport of pilocarpine from the cornea to the anterior chamber is controlled by corneal endothelium 4. Pilocarpine depot somewhere in the cornea
- 45. Transcorneal permeation depends upon nature of penetrant moleculle The drug must have optimum patition coeft to get absorbed Pilocarpine reach the max after 5min in epithelium and cornea the decline follow biexponential In case of aq.humour and stroma the peak pt is achieved after 20 min and then declines Pilocarpine elimination rate const is same in cornea and aq.humour Corneal epithelium is the main barrier to the transcorneal permeaton
- 46. PHARMACOKINETICS OF TRANSCORNEAL PILOCARPINE PERMEATION Tear flow qT Precorneal k Epithelial kaE Corneal k eE a Stroma drug pool surface epithelium kas ke kaAH k es Knl kem Aq.humour Conjuctiva Nasolacrimal keAH drainage metabolism Elimination
- 47. qt = normal production rate of tear (.66 micro lt) knl =1st order rateof elimination through nasolacrimal drainage Kc = rate const for conjuctival uptake Ka = rate const for epithelial uptake kaE = rate const for absorption into epithelium keE = rate const for elimination from epithelium kaS = rate const for absorption into stroma epithelium keS = rate const for elimination from stroma kaAH=rate const for absorption into aq. humour k AH= rate const for elimination from aq. humour
- 48. Considering eye as two major compartment Precorneneal area Aq. Humour Rate of pilocarpine disappears from the precorneal compartment as dCT = -qT CT – KpSc/ hc (CT – CAH) dt VDe –Knlt + V0 Rate of appearance of pilocarpine in the aq humour dCAH KpSc (C – C ) - K CAH = T AH eAH dt VAH hc VAH
- 49. CT = drug conc in tear fluid CAH = conc of drug in aq.humor KP =specific transcorneal permeation rate (3.675 *10 -4 ) Knl =(0.25 +0.0113 VD ) min-1 SC = surface area of cornea (2 cm2 ) H = thickness of cornea (0.035cm) V0 = normal residant tear volume (7.5 ) VD = drop size of drug soln instilled VPC = volume of drug pool in precorneal area after instillation of eye drops VAH = volume of aq.humor
- 50. Elimination of pilocarpine follows biphasic pattern alpha phase has an apparent absorption rate 0.50 /min apparent elimination rate 0.06 / min Beta phase apparent elimination rate – 0.016/ min Stroma endothelium consist one absorption and one elimination phase which has similar pk as of aq.humour i.e kaAH = keS Hence endothelial layer doesnot act as a barrier for the moment of drug from cornea to aq.humor
- 51. Epithelium of cornea is the rate controlling barrier in the transcorneal permeation of pilocarpine Once the drug reaches the precorneal area it undergo disposition by various ways Due to the protein binding also the bioavailability decreases
- 52. CONTROLLED OCULAR PILOCARPINE DELIVERY BY OCUSERT SYSTEM The ocusert system is an oval flexible ocular insert that consists of a core reservoir made from complexation of pilocarpine with alginic acid sandwiched between two sheets of a transparent, lipophilic rate-controlling membrane of ethylene- vinyl acetate copolymer. When inserted it released through the rate-controlling membrane according to a zero-order kinetics process. 52
- 53. EXPLAIN MATHEMATICALLY BY EQUATION: (dQ/ dt)r = Dp km (CR-CT)/hm Where, dQ/dt = release rate of pilocarpine from a unit surface area of the ocusert system. Dp = diffusivity of plocarpine from in the ethylene-vinyl acetate co-polymer membrane with thickness hm Km = partition coefficient of pilocarpine towards the membrane. (CR-CT) = difference in pilocarpine concentration between the pilocarpine reservoir in the medicated core (CR) and the tear fluid (CT).
- 54. Ifan infinite sink condition is maintained in the ocular cavity that is CR>>CT then equation is simplified. (dQ/ dt)r = Dp km Cs/hm. The release rate of pilocarpine from the ocusert system should remain constant until the drug concentration in the reservoir CR drops off below the pilocarpine saturation level Cs.
- 55. Membrane permiability can be increased by adding dipthalate during fabrcation The release rate is not efeected by the ca inhibitor ephenephrine , NSAIDS etc . 8-10 times more efficient than 2% eye drops Increased patient complience Decreased dosing frequency Sustain release Less side effects (decreased myopia )
- 56. REFERENCES Vyas S P, Roop K Khar. Controlled Drug Delivery Concepts and Advances. 1 st Edition Vallabh Prakashan Delhi.2002. Chien Y. W, Novel Drug Delivery System. Marcell Decker Inc., Ed.1992. Jain N. K, Advances In Controlled & Novel Drug Delivery, CBS Publication, Ed.2003. www.soople.com www.google.com 56
- 57. Thank You